Heme iron polypeptide in treatment of anemia inpregnancy
Abstract
Introduction: Treatment of iron deficiency anemia with non-heme iron salts still remains a challenge. The negative influence of various dietary elements and medications slows the absorption of iron from iron salts which makes it less bio-available, the side effects associated with iron salts, results in poor compliance. But Heme Iron Polypeptide claims to overcome the short comings of the non – heme oral iron.
Materials and Methods: This prospective clinical study was done on patients of SSG Hospital, Baroda; 100 mild to moderate anemia pregnant patients between 16 to 28 weeks were included. The primary outcome of interest was mean maternal hemoglobin and serum ferritin levels at the end of treatment. Secondary outcomes were treatment related side effects and compliance.
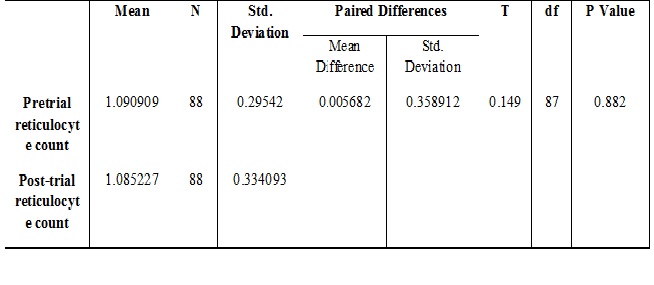
Results: There was significant rise in hemoglobin [mean difference (MD), 1.05841; p< 0.001] and serum ferritin [mean difference (MD) 7.3715, p, 0.001]. No side effects and good compliance with Heme Iron Polypeptide.
Conclusion: Heme Iron Polypeptide is promising supplementary treatment for iron deficiency anemia in pregnancy as there was significant rise in hemoglobin and other iron indices which are comparable to oral iron salts. Heme Iron Polypeptide is a better source of iron for iron deficiency anemia in pregnancy, as it has less side effects especially gastrointestinal side effects, because of which it has better compliance.
Downloads
References
2. Lieu PT, HeiskalaM, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001 Feb-Apr;22(1-2):1-87.[pubmed]
3. Soyano A, Gómez M. [Role of iron in immunity and its relation with infections]. Arch LatinoamNutr. 1999 Sep;49(3 Suppl 2):40S-46S.[pubmed]
4. Naigamwalla DZ, Webb JA, Giger U. Iron deficiency anemia. Can Vet J. 2012 Mar;53(3):250-6.[pubmed]
5. Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008 Nov;121(11):943-8. doi: 10.1016/j.amjmed.2008.07.012.[pubmed]
6. Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011 May;4(3):177-84. doi: 10.1177/1756283X11398736.[pubmed]
7. Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring HarbPerspect Med. 2013 Jul 1;3(7). pii: a011866. doi: 10.1101/cshperspect.a011866.[pubmed]
8. Nagaraju SP, Cohn A, Akbari A, et al. Heme iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: a randomized controlled trial. BMC Nephrol. 2013 Mar 20;14:64. doi: 10.1186/1471-2369-14-64.[pubmed]
9. Park K, Kim H, Han M, et al. Heme iron polypeptide polymer with high iron content as an ideal iron supplement. Journal of Food Biochemistry.2010; 34(4); 896-904. doi.org/10.1111/j.1745-4514.2010.00342.x
10. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of heme iron (blood peptonates) for the proposed uses as a source of iron added for nutritional purposes to foods for the general population, including food supplements. EFSA Journal.2010;8(4):1585.doi: 10.2903/j.efsa.2010.1585.
11. Heme Iron Polypeptide (Proferrin) versus Oral and Injectable Iron Products for the Treatment of Anemia. https://www.cadth.ca>media>pdf>htis
12. Pal, B., Deshpande, H., Sundari, T., Biniwale,P. Shah, K. , Goel, S. ,Khurana, A. , Qamra, A., Motlekar, S. and Barkate, H. Heme Iron Polypeptide in Iron Deficiency Anemia of Pregnancy: Current Evidence. Open journal of Obstetrics and Gynecology,2017;7,420-431. doi:10.4236/ojog.2017.74044
13. SyalNeeru, N. Sreekumaran Nair and Lavanya Rai; Iron Sucrose Versus Oral Iron Therapy in Pregnancy Anemia . Indian Journal of Community Medicine.2012Oct-Dec;37(4):214-218. doi:10.4103/0970-0218.103467.[pubmed]
14. Barraclough KA, Noble E, Leary D, et al. Rationale and design of the oral HEMe iron polypeptide Against Treatment with Oral Controlled Release Iron Tablets trial for the correction of anaemia in peritoneal dialysis patients (HEMATOCRIT trial). BMC Nephrol. 2009 Jul 28;10:20. doi: 10.1186/1471-2369-10-20.[pubmed]
15. Barraclough KA, Brown F, Hawley CM, et al. A randomized controlled trial of oral heme iron polypeptide versus oral iron supplementation for the treatment of anaemia in peritoneal dialysis patients: HEMATOCRIT trial. Nephrol Dial Transplant. 2012 Nov;27(11):4146-53. doi: 10.1093/ndt/gfs372. Epub 2012 Sep 7.[pubmed]
16. Abdelazimn ,I.a,Abu-Faza,M.,Eliaa, A.A.M, Othman, H.S, Alsharif, D.A and Elsawah ,W.F. Heme Iron Polypeptide (Proferrin –ES) versus Iron Saccharate Complex (Ferrosac) for Treatment of Iron Deficiency Anemia during Pregnancy .Acta Med Int 2017:4,56-61.doi:10.5530/ami.2017.4.11.
17. al-Momen AK, al-Meshari A, al-Nuaim L, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J ObstetGynecolReprod Biol. 1996 Nov;69(2):121-4.[pubmed]
18. Frykman E, Bystrom M, Jansson U, et al. Side effects of iron supplements in blood donors: superior tolerance of heme iron. J Lab Clin Med. 1994 Apr;123(4):561-4.[pubmed]
19. Nam,T.,Shim,J.Y.,Kim,B.,Rah,S.Y.,Park,K.,Kim,S.,Mun,E.G.,Jeong,Y.J.,Han,M.K.,Cha,Y.S.,Chae,S.W.,Im,M.J. and Kim, U.H. Clinical Study on the Iron Absorption from Heme –Iron Polypeptide and Non heme –Iron. Nutritional Sciences,2006; 9,295-300.
20. Wish JB, Fourtner P, Ghaddar S, Moore GM. The biological and economic value of oral organic iron in maintenance dialysis. Nephrol News Issues. 2002 Mar;16(4):32-3, 37-9.[pubmed]
21. Pizarro F, Olivares M, Hertrampf E et al. Heme-iron absorption is saturable by heme-iron dose in women. J Nutr. 2003 Jul;133(7):2214-7.[pubmed]
22. Björn-Rasmussen E, Hallberg L, Isaksson B, Arvidsson B. Food iron absorption in man. Applications of the two-pool extrinsic tag method to measure heme and nonheme iron absorption from the whole diet. J Clin Invest. 1974 Jan;53(1):247-55. DOI:10.1172/JCI107545.[pubmed]
23. Monsen ER. Iron nutrition and absorption: dietary factors which impact iron bioavailability. J Am Diet Assoc. 1988 Jul;88(7):786-90.[pubmed]
24. Ekman M, Reizenstein P. Comparative absorption of ferrous and heme-iron with meals in normal and iron deficient subjects. Z Ernahrungswiss. 1993 Mar;32(1):67-70.
Copyright (c) 2018 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative



















 Therapoid
Therapoid

