Evaluation of Pregnancy Outcome in Severe Preeclampsia
Sultana S1*, Siddique MAB2, Hoque S3, Shahriar SM4, Karim K5
DOI:https://doi.org/10.17511/joog.2024.i01.02
1* Salma Sultana, Junior Consultant, Obst and Gaynae, 250 Bedded General Hospital, Thakurgaon, Bangladesh.
2 Md Abu Baker Siddique, Assistant Professor, MS Orthopaedic, M Abdur Rahim Medical College, Dinajpur, Bangladesh.
3 Smrity Hoque, Junior Consultant, Obst and Gaynae, Current Charge Baliadangi Upazila Health Complex, Thakurgaon, Bangladesh.
4 Shihab Mahmud Shahriar, Junior Consultant, Surgery, 250 Bedded General Hospital, Thakurgaon, Bangladesh.
5 Khadiza Karim, Medical Officer, Obst and Gaynae, 250 Bedded General Hospital, Thakurgaon, Bangladesh.
Background: Hypertensive disorders represent the most common medical complication of pregnancy affecting between 7 to 15 percent of all gestation and account for approximately a quarter of all antenatal admission. In Bangladesh, the incidence of this killer disease is still high and it is the third major cause of maternal death in our country.
Objective: To evaluate pregnancy outcomes in severe preeclampsia.
Methods: The data were collected through the active participation of the patients' interviews by the performed proforma of data collection sheet and then data were gathered, decorated, and tabulated after data cleaning and edition. Then the results were found and they were tested by the Student's unpaired t-test (quantitative data) and chi-square test (qualitative data) to see their level of significance i,e p-value which was set as the cutoff level at <0.05. So if the p-value is >0.05 the results are not significant.
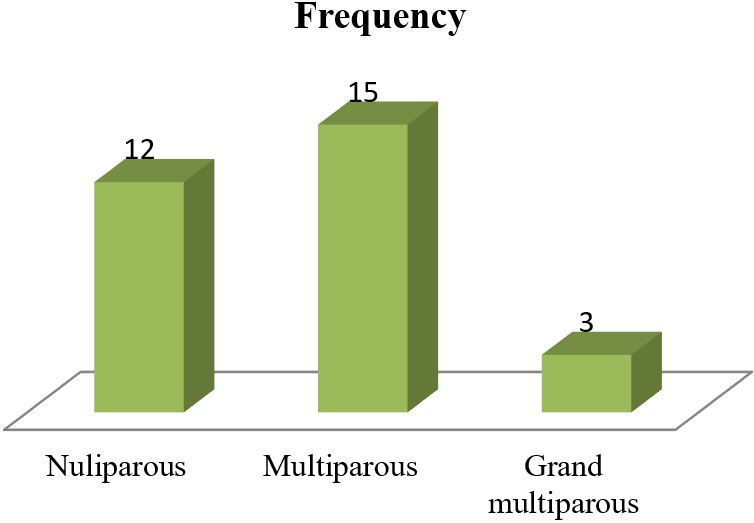
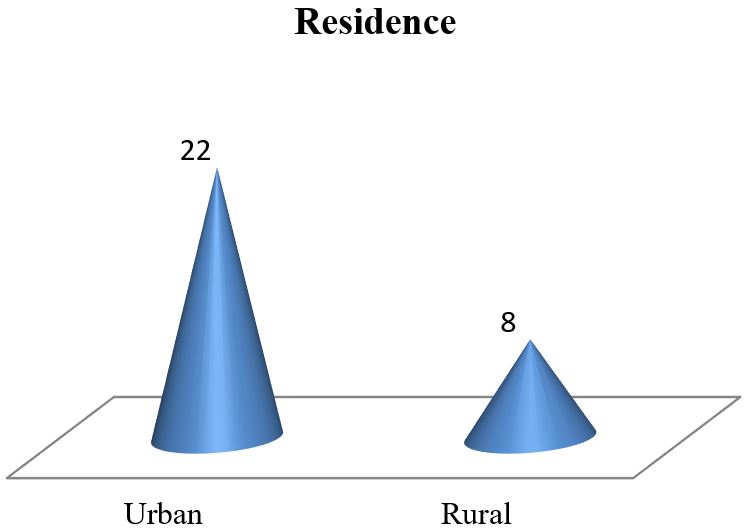
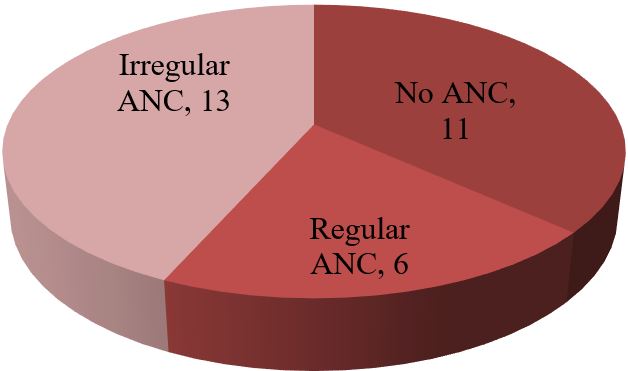
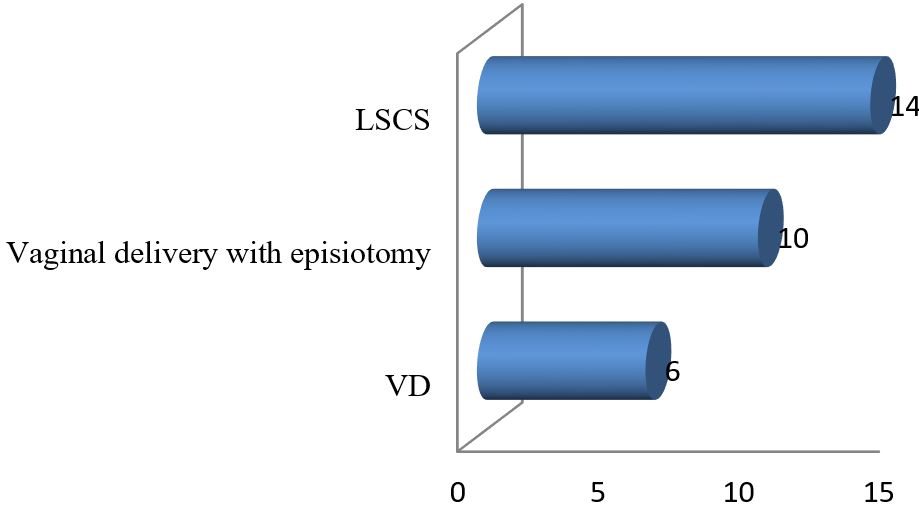
Results: The data analysis of 30 patients yielded the following results. The mean age of 30 mothers was 27.65 (± 5.85) years. The maximum 19 (63.33%) patients were from the 20-34 years age group. Among the 30 mothers with severe preeclampsia, 12(40%) were primiparous and the rest 18 (60%) were multiparous. To ensure safe delivery 14(46.67%) mothers adopted LSCS whereas 10(33.33%) adopted vaginal delivery with episiotomy. The rest 6 (20%) underwent VD. Regular ANC ensured only 6 (20%) respondents whereas the rest 80% replied that they were either irregular ANC or no ANC. The most common complications mothers faced were abruptio placenta (28.57%) followed by convulsion (14.28%) and HELLP syndrome (9.52%). Among the babies, 5% showed APGAR score above 7 at 1st minute whereas 58% showed the same score at 5 minutes.
Conclusion: In conclusion, it may be assumed that though various fetal and maternal morbidities may occur in the case of severe preeclampsia mortality is seen only in the case of neonates. Maternal mortality has almost disappeared from our perspective in the case of severe pre-eclampsia.
Keywords: Evaluation, Pregnancy, Outcome, Severe Preeclampsia
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Junior Consultant, Obst and Gaynae, 250 Bedded General Hospital, Thakurgaon, , Bangladesh. Email: |
Sultana S, Siddique MAB, Hoque S, Shahriar SM, Karim K, Evaluation of Pregnancy Outcome in Severe Preeclampsia. Obs Gyne Review J Obstet Gynecol. 2024;10(1):9-15. Available From https://obstetrics.medresearch.in/index.php/joog/article/view/168 |


 ©
©