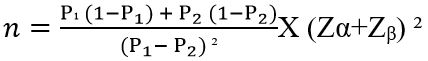Comparison of Maternal and Perinatal Outcome Of Gestational Diabetes Mellitus With And Without Preeclampsia Patient
Begum N1*, Shelly SJ2, Karim A3, Sultan S4, Biswas J5, Ferdous M6, Islam N7
DOI:https://doi.org/10.17511/joog.2024.i01.07
1* Nurjahan Begum, Assistant Registrar MS, Obstetrics and Gynecology, District Hospital, Sherpur, Bangladesh.
2 Samsad Jahan Shelly, Professor and Head, Department of Obstetrics and Gynecology, BIRDM General Hospital Shagunbagicha, Dhaka, Bangladesh.
3 Abdul Karim, Assistant Professor, Department of ENT, Jamalpur Medical College Hospital, Jamalpur, Bangladesh.
4 Sabiha Sultan, Consultant MS, Obstetrics and Gynecology, Habib Hospital Ashulia, Dhaka, Bangladesh.
5 Joya Biswas, Medical Officer MS, Obstetrics and Gynecology, National Institute of Burn and Plastic Surgery, Dhaka, Bangladesh.
6 Morsheda Ferdous, Consultant MS, Obstetrics and Gynecology, Islami Bank Hospital Cardiac Centre Mirpur, Dhaka, Bangladesh.
7 Nahida Islam, RS MS, Obstetrics and Gynecology, Tangail Medical College Hospital, Tangail, Bangladesh.
Background: Gestational diabetes mellitus (GDM) and preeclampsia share many risk factors, e.g., gestational weight gain. Gestational diabetes mellitus (GDM) and preeclampsia are two dangerous pregnancy complications and their coexistence further increases adverse maternal and perinatal outcomes.
Objective: This study was carried out for comparison of maternal and perinatal outcomes of gestational diabetes mellitus with and without preeclampsia patients.
Methods: It was a cross-sectional analytical study. Patients were divided into 2 groups: group I (n=75) included GDM with preeclampsia and group II (n=75) included GDM without preeclampsia. Data was collected by history taking, examinations and required investigations and recorded in a predesigned data collection instrument. Data were processed and analyzed using computer software SPSS version 22.
Results: The majority, 66.7% among the respondents of group I did not have anemia, 30.7% had mild and only 2.7% had moderate anemia. 86.7% of the respondents of group II did not have anemia, 12.0% had mild and only 1.3% had moderate anemia which was statistically significant (p<0.05). Mean BMI in both groups I (31.87± 5.93) and II (31.16 ± 4.72) was not statistically significant (p≥0.05). Of all (100.0%) of group I and group II most of the respondents, 90.7% had LUCS and none of group I, only 9.3% in group II had NVD both were statistically significant (p=.013). A non-significant difference was observed in regards to maternal outcome where group I, had 16.0% oligohydramnios, 4.2% polyhydramnios and 1.3%, PPH, in group II had 12%, oligohydramnios, 4.2% polyhydramnios and none of PPH. In group I majority, 64.0% had preterm delivery and in group II, 41.3% had preterm delivery which was statistically significant. In group I, 5.3% and none of group II had an IUD which was statistically significant (p<0.05) no significant difference was observed in regards to asphyxia whereas in group I asphyxia of babies was higher (10.7%) than that of group II, 4.0%.
Conclusion: Preeclampsia is one of the leading causes of maternal and perinatal morbidity and mortality worldwide. In GDM with preeclampsia LUCS, preterm delivery and IUD were higher in comparison to patients in GDM without preeclampsia.
Keywords: Gestational Diabetes Mellitus, Maternal Mortality, Maternal Outcome, Neonatal Outcome
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Registrar MS, Obstetrics and Gynecology, District Hospital, Sherpur, , Bangladesh. Email: |
Begum N, Shelly SJ, Karim A, Sultan S, Biswas J, Ferdous M, Islam N, Comparison of Maternal and Perinatal Outcome Of Gestational Diabetes Mellitus With And Without Preeclampsia Patient. Obs Gyne Review J Obstet Gynecol. 2024;10(1):49-57. Available From https://obstetrics.medresearch.in/index.php/joog/article/view/173 |


 ©
©