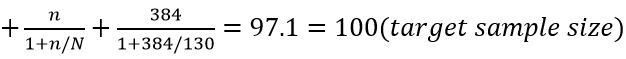Comparative Study Between Tablet Labetalol and Methyldopa In Treatment Of Pregnancy Induced Hypertension
Sultana A1*, Begum T2, Siddique MAB3, Akter A4, Patuary S5, Nasrin UT6
DOI:10.17511/joog.2024.i01.04
1* Afroza Sultana, Medical Officer, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gazipur, Bangladesh.
2 Tashrin Begum, Senior Consultant, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gagipur, Bangladesh.
3 Md Abu Bakar Siddique, Assistant Professor, Department of Surgery, Shaheed Tajuddin Ahmad Medical College Hospital, Gazipur, Bangladesh.
4 Ahsana Akter, Senior Consultant, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gagipur, Bangladesh.
5 Sakila Patuary, Junior Consultant, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gagipur, Bangladesh.
6 Ummae Tania Nasrin, Resident Surgeon, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gagipur, Bangladesh.
Background: Pregnancy-induced hypertension is one of the most significant health problems in pregnancy. This is the 2nd most common obstetrics cause of maternal death in Bangladesh. It is the leading cause of infant morbidity and mortality.
Methodology: A randomized controlled trial was carried out among 100 pregnant women with pregnancy-induced hypertension (PIH) attending the Obstetrics & Gynaecology Department at Dhaka Medical College & Hospital from November 2010 to April 2011. 50 patients treated with tab. Labetalol (Group A) and 50 treated with tab. Methyldopa (Group B).
Results: Findings of the study showed mean age, gestational age and occupation did not differ significantly variation between Labetalol (group A) and Methyldopa (group B). Among 36% had gestational HTN, 62% had preeclampsia and 2% had eclampsia in group A. On the other hand in group B 32% had gestational HTN, 64% had preeclampsia and 4% had eclampsia. Among 23 patients in group A (46%) went in normal whereas (32%) went in normal vaginal delivery in group B. Maternal morbidity was more in Group B than in Group A. The most common morbidity was pulmonary oedema (6%) in group A and 14% had pulmonary oedema in group B. At the time of discharge, in the group, 85.41% of patients had normal blood pressure and 95.83% of patients had no proteinuria. Whereas in group B 80.43% had normal blood pressure and 91.30% had no proteinuria. The incidence of stillbirth was higher in the methyldopa group (group B). Low birth weight was lower in the labetalol group (group A).
Conclusion: It concluded that labetalol is more advantageous than methyldopa in terms of better and quicker control of blood pressure. The chances of normal vaginal delivery were greater in the labetalol group than in the methyldopa group.
Keywords: Pregnancy Induced Hypertension, Methyldopa, Labetalol
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Medical Officer, Obstetrics and Gynae, Shaheed Ahsan Ullah Master General Hospital, Tongi, Gazipur, Bangladesh. Email: |
Sultana A, Begum T, Siddique MAB, Akter A, Patuary S, Nasrin UT, Comparative Study Between Tablet Labetalol and Methyldopa In Treatment Of Pregnancy Induced Hypertension. Obs Gyne Review J Obstet Gynecol. 2024;10(1):25-31. Available From https://obstetrics.medresearch.in/index.php/joog/article/view/170 |


 ©
©