The effectiveness of two different doses letrozole in ovulation induction – a comparative study
Gayam S.1*, Sajja B.2, Shashikala M.3, Neelima C.4, Rani G.5
DOI: https://doi.org/10.17511/joog.2020.i04.01
1* Susheela Gayam, Head of Department, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India.
2 Bhargavi Sajja, Registrar, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India.
3 Mary Shashikala, Senior Consultant, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India.
4 Neelima C, Senior Consultant, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India.
5 Geeta Rani, Junior Consultant, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India.
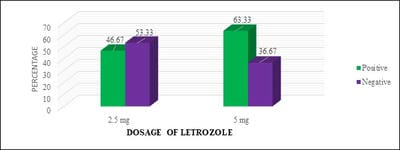
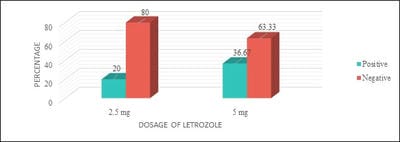
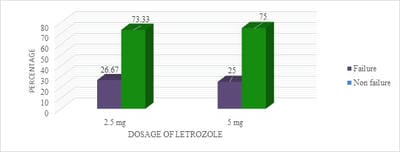
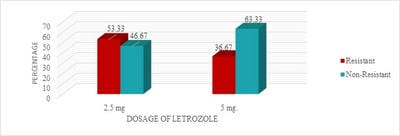
Introduction: Letrozole is a third-generation selective aromatase inhibitor and an effective agent for ovulation induction. Even though it was used in Clomiphene Citrate failure cases, Clomiphene Citrate resistant women, for Intra-Uterine insemination, and also for mild stimulation for IVF/ICSI cases it has not gained its importance as a first-line drug for ovulation induction. Objective: To study the effectiveness of two different doses of Letrozole in ovulation induction and pregnancy rates and to calculate the proportion of Letrozole resistant and failure cases. Materials and Methods: One year Prospective, observational study was conducted on 120 study participants who were divided into two groups and were given either 2.5 or 5 mg of Letrozole respectively. Results: It was observed that 28 (46.67%) of 60 study participants in group I and 38 (63.33%) of 60 study participants in group II had ovulated. Conclusion: It is observed that there was no significant statistical difference in ovulation rates, Letrozole failure, and resistance rates in both groups. Though the endometrial growth was comparable in both study groups, it was observed that there was better follicular growth in study group II.
Keywords: Letrozole, Two different doses, Ovulation Induction, Letrozole Failure Rates, Letrozole Resistance Rates and Pregnancy Rates
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Head of Department, Department of Obstetrics and Gynaecology, Vijay Marie Hospital and Educational Society, Hyderabad, Telangana, India. Email: |
Gayam S, Sajja B, Shashikala M, Neelima C, Rani G. The effectiveness of two different doses letrozole in ovulation induction – a comparative study. Obs Gyne Review J Obstet Gynecol. 2020;6(4):72-78. Available From https://obstetrics.medresearch.in/index.php/joog/article/view/115 |


 ©
©